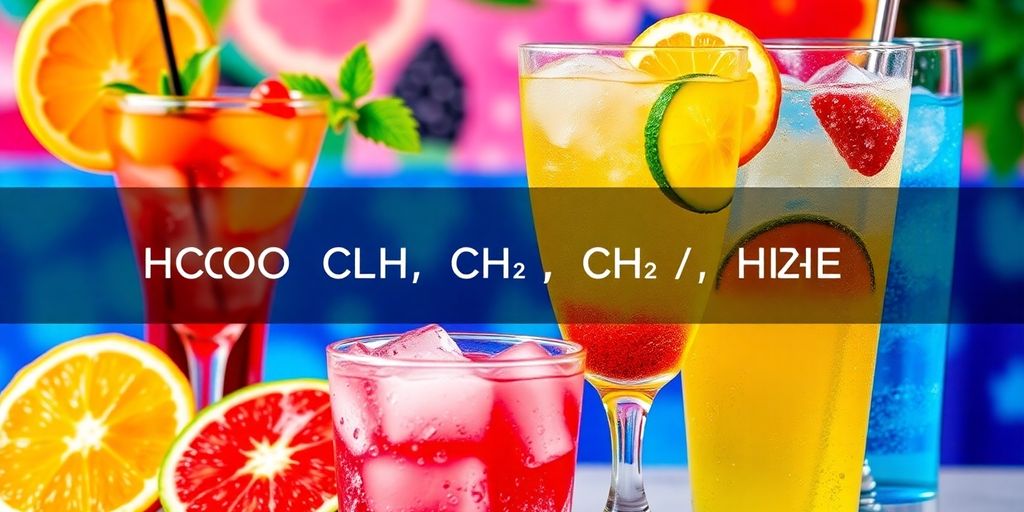
When you sip on your favorite drinks, you might not think about the chemistry behind them. Yet, compounds like HCOOCH, CH2, and H2O play essential roles in the flavors and experiences we enjoy. Understanding these chemicals can give us insight into not only how our beverages are made but also why they taste the way they do. Let’s break down these components and see how they come together to create the drinks we love!
Key Takeaways
- HCOOCH is an important organic compound used in various beverages.
- CH2 serves as a building block in many organic molecules, influencing stability and functionality.
- Water (H2O) is crucial for flavor extraction and chemical reactions in drinks.
- The interaction between HCOOCH and H2O can enhance or alter the taste of beverages.
- Understanding these compounds can lead to better choices in both health and flavor.
Introduction to HCOOCH, CH2, and H2O
Alright, let’s talk about some really important stuff: HCOOCH, CH2, and H2O. You might be thinking, “Okay, chemistry, great…” but stick with me! These little guys are way more interesting than they sound, especially when it comes to your favorite drinks. We’re going to break down what they are, what they do, and why they matter in the world of beverages. Trust me, it’s going to be fun!
Think of it this way: HCOOCH, CH2, and H2O are like the secret ingredients that make your drinks taste the way they do. They’re involved in everything from the flavor to the overall experience. So, grab your favorite beverage (water totally counts!), and let’s get started.
Understanding these compounds isn’t just about memorizing chemical formulas. It’s about appreciating the science behind everyday pleasures. It’s about knowing why your soda fizzes, why your juice tastes sweet, and why your coffee smells so darn good in the morning.
Understanding the Chemical Structure of HCOOCH
Let’s break down the chemical structure of HCOOCH, also known as methyl formate. It’s not as scary as it sounds! Understanding its structure is key to understanding its properties and how it interacts with other molecules.
Think of it like this: HCOOCH is built from smaller pieces. It’s an ester, which means it’s formed from an alcohol and a carboxylic acid. In this case, it’s derived from formic acid and methanol. The arrangement of atoms gives it specific characteristics.
Here’s a simple breakdown:
- It has a carbonyl group (C=O), which is a carbon atom double-bonded to an oxygen atom.
- It’s connected to a methoxy group (-OCH3).
- The carbon atom is also bonded to a hydrogen atom.
The way these atoms are arranged affects how HCOOCH behaves. For example, the carbonyl group makes it polar, which influences its solubility and reactivity.
Because of its structure, HCOOCH has some interesting properties. It’s a volatile liquid, meaning it evaporates easily. It’s also a good solvent for some organic compounds. This makes it useful in a variety of applications, from flavorings to industrial solvents. It’s also related to formic acid, which is a vital chemical intermediate.
The Role of CH2 in Organic Compounds
The methylene group, CH2, is a fundamental building block in organic chemistry. It’s more than just a simple combination of carbon and hydrogen; it’s a versatile component that influences the structure and reactivity of countless molecules. Understanding its role is key to understanding CH2 in food science and the behavior of many compounds we encounter daily.
- It acts as a bridge, connecting different parts of a molecule.
- It can alter the shape and stability of a compound.
- It influences how a molecule interacts with others.
The presence of CH2 groups can significantly affect a molecule’s properties, such as its boiling point, melting point, and solubility. These seemingly small changes can have big impacts on how a substance behaves and how we use it.
Think about fatty acids, for example. The long chains of CH2 groups are what make them hydrophobic, meaning they don’t mix well with water. This property is essential for the formation of cell membranes and the storage of energy in our bodies.
The Importance of H2O in Beverage Chemistry
Water is often overlooked, but it’s the most important ingredient in almost every drink. It’s not just a filler; it actively shapes the taste, texture, and overall experience of what we consume. Understanding water’s role in drink chemistry is key to appreciating the nuances of your favorite beverages. The quality of water used can drastically alter the final product, influencing everything from the clarity of a spirit to the chemical composition of beverages.
How Water Affects Flavor Profiles
Water isn’t just a neutral base; it’s an active participant in flavor development. The minerals present in water can either enhance or detract from the intended taste profile of a beverage. For example, water with high mineral content might make a beer taste bitter, while softer water might allow the malt flavors to shine through. The pH level of water also plays a role, affecting the perceived acidity and sweetness of a drink.
- Mineral content
- pH level
- Source of water
The Interaction of HCOOCH and H2O in Drinks
When HCOOCH (methyl formate) and H2O (water) are combined in a beverage, interesting chemical interactions can occur. While HCOOCH isn’t a common ingredient, understanding its potential behavior helps illustrate the broader principles of beverage chemistry. The presence of water can cause HCOOCH to undergo hydrolysis, breaking it down into formic acid and methanol. This reaction can alter the flavor profile and potentially impact the safety of the drink, depending on the concentrations involved. The rate of this reaction is influenced by factors like temperature and pH.
The interplay between water and other compounds in a drink is complex. Water acts as a solvent, facilitating reactions and influencing the perception of flavors. It’s a dynamic component that deserves careful consideration in beverage formulation.
Common Beverages Containing HCOOCH, CH2, and H2O
Alcoholic Beverages and Their Chemical Components
Alcoholic drinks are complex mixtures, and understanding their chemistry can really change how you see them. Ethanol (CH3CH2OH), a close relative to our CH2 group, is the star of the show, but it’s the supporting cast of organic compounds in drinks that gives each beverage its unique character. Think about it: wine gets its flavor from esters and tannins, while beer relies on hops and malt-derived compounds. Even the simplest vodka is more than just ethanol and water; trace amounts of other alcohols and flavor compounds contribute to its overall profile. These components are formed during fermentation and aging, and they interact in ways that are still being studied. The presence of water (H2O) is also critical, as it acts as a solvent and influences the perception of flavor and mouthfeel.
Non-Alcoholic Drinks: A Closer Look
Non-alcoholic beverages also have a fascinating chemistry. Carbonated soft drinks, for example, rely on carbonic acid (formed from CO2 and H2O) for their characteristic fizz. Juices contain a variety of sugars, acids, and volatile compounds that contribute to their flavor. Even something as simple as tea or coffee is a complex mixture of hundreds of different chemicals, including caffeine, tannins, and various aromatic compounds. The effects of HCOOCH in beverages are less direct here, but similar esters can contribute to fruity or floral notes in some drinks. Water, again, plays a vital role, not only as a solvent but also in influencing the extraction of flavors from the source ingredients.
It’s easy to overlook the chemistry in our everyday drinks, but it’s there, influencing everything from taste to texture. Understanding these chemical interactions can help us appreciate the complexity and artistry involved in creating our favorite beverages.
Health Implications of HCOOCH, CH2, and H2O Consumption
It’s important to understand how the chemicals in our drinks affect our health. While many beverages are safe in moderation, the components HCOOCH, CH2, and H2O can have varying impacts depending on concentration and individual sensitivities. Let’s take a closer look.
Alcoholic Beverages and Their Chemical Components
Alcoholic drinks contain ethanol (a type of alcohol), which is formed through fermentation. This process also produces other compounds, including esters like HCOOCH. While ethanol itself has well-known effects on the body, such as impaired judgment and liver damage with excessive consumption, the other components can also play a role. For example, some esters contribute to the flavor profile but might also cause allergic reactions in sensitive individuals. It’s all about balance and moderation.
Non-Alcoholic Drinks: A Closer Look
Even non-alcoholic beverages contain these chemicals. Carbonated drinks, for instance, contain H2O and often have added sugars or artificial sweeteners. The CH2 group is a fundamental part of many organic molecules, including these sweeteners. While H2O is essential for life, excessive sugar intake can lead to health problems like weight gain and type 2 diabetes. It’s crucial to read labels and be mindful of the ingredients and quantities you’re consuming.
It’s worth noting that the health effects of these chemicals are often dose-dependent. Small amounts might be harmless or even beneficial, while large amounts can be detrimental. Individual factors, such as genetics and overall health, also play a significant role in how these chemicals affect each person.
Here’s a quick rundown of potential health implications:
- HCOOCH (Esters): Potential allergens, contribute to flavor.
- CH2 (Methylene Group): Part of sugars and sweeteners, impacts metabolic processes.
- H2O (Water): Essential for hydration, but excessive intake can dilute electrolytes.
Understanding the chemical structure of CH2 H2O and other compounds can help you make informed choices about what you drink.
Future Trends in Beverage Chemistry
The beverage industry is always changing, and the future looks pretty interesting. We’re seeing new technologies and a bigger focus on what consumers want, which is pushing beverage chemistry in some cool directions. It’s not just about making something taste good anymore; it’s about making it healthier, more sustainable, and even personalized.
Alcoholic Beverages and Their Chemical Components
Alcoholic drinks are getting a makeover, thanks to chemistry. Think about it: we’re seeing more research into how different fermentation processes affect flavor and aroma. Scientists are exploring new yeast strains and enzymes to create unique flavor profiles and reduce unwanted byproducts like fusel alcohols (which can cause hangovers). Also, there’s a growing interest in using local and sustainable ingredients, which requires a deeper understanding of how these ingredients interact chemically during brewing or distillation. This could lead to some really interesting regional variations in your favorite drinks. For example, sustainable ingredients are becoming more popular.
Non-Alcoholic Drinks: A Closer Look
Non-alcoholic beverages are also getting a lot of attention. People want healthier options, so companies are looking at ways to reduce sugar and artificial additives. This involves some clever chemistry, like using natural sweeteners that don’t spike blood sugar or finding plant-based extracts that can mimic the taste and mouthfeel of sugar.
Here are some trends:
- Sugar Reduction: Developing natural sweeteners and flavor enhancers to reduce sugar content without sacrificing taste.
- Functional Ingredients: Incorporating vitamins, minerals, and antioxidants for added health benefits.
- Personalized Beverages: Creating drinks tailored to individual needs and preferences using data-driven formulations.
The future of non-alcoholic drinks is all about customization and health. Imagine a drink that’s perfectly tailored to your specific dietary needs and taste preferences. That’s where beverage chemistry is headed.
It’s a pretty exciting time for beverage chemistry, and I can’t wait to see what new drinks end up on store shelves in the next few years.
Conclusion: The Chemistry of Enjoyment
It’s pretty amazing when you think about it – the drinks we enjoy so much are really just a bunch of chemical reactions happening in a glass. From the simplest glass of water to the most complex cocktail, HCOOCH, CH2, and H2O are all playing their parts. Understanding these molecular interactions can really change how you see your favorite beverages.
So, next time you’re sipping on something tasty, take a moment to appreciate the chemistry at work. It’s not just about quenching your thirst; it’s about experiencing a carefully crafted blend of molecules that come together to create something truly enjoyable.
- The interplay of these compounds affects taste.
- They influence the drink’s texture.
- They even impact how our bodies process what we’re drinking.
Wrapping It Up
In conclusion, getting to know HCOOCH and CH2 H2O is pretty important if you’re curious about chemistry and how it fits into our lives. This compound isn’t just some random formula; it plays a big role in many everyday products and processes. From the food we eat to the medicines we take, its unique properties make it super useful. Plus, understanding how it reacts with other substances helps us see its value in both science and industry. So, whether you’re a student, a professional, or just someone interested in the science behind your favorite drinks, knowing about HCOOCH and CH2 H2O can really broaden your perspective on the world around you.
Frequently Asked Questions
What is HCOOCH?
HCOOCH, also known as methyl acetate, is a chemical compound made of carbon, hydrogen, and oxygen. It is commonly used in various drinks and products.
How does CH2 fit into organic compounds?
CH2, or methylene, is a small part of many organic compounds. It helps connect other molecules and is important for their structure.
Why is H2O important in drinks?
H2O, or water, is essential for life and plays a big role in the taste and quality of drinks. It helps mix other ingredients and affects flavor.
Can you give examples of drinks containing HCOOCH?
Yes! Many alcoholic beverages, like certain wines and spirits, contain HCOOCH. Some flavored non-alcoholic drinks may also include it.
What health effects can HCOOCH and CH2 have?
In small amounts, HCOOCH and CH2 are generally safe in drinks. However, excessive consumption can lead to health issues, so moderation is key.
How does water affect the flavor of drinks?
Water can enhance or dull flavors in drinks. The right amount of water can bring out the best taste, while too much can make it bland.
What are some common misconceptions about HCOOCH and CH2?
Many people think all carbon compounds are the same, but HCOOCH and CH2 have unique properties that make them different from others.
What future trends are there in beverage chemistry?
Researchers are exploring new ways to use compounds like HCOOCH and CH2 to create healthier, tastier drinks, and improve sustainability in production.